Stem Cells – a Disruptive „Technology“ Changing Medical Practice and Thinking
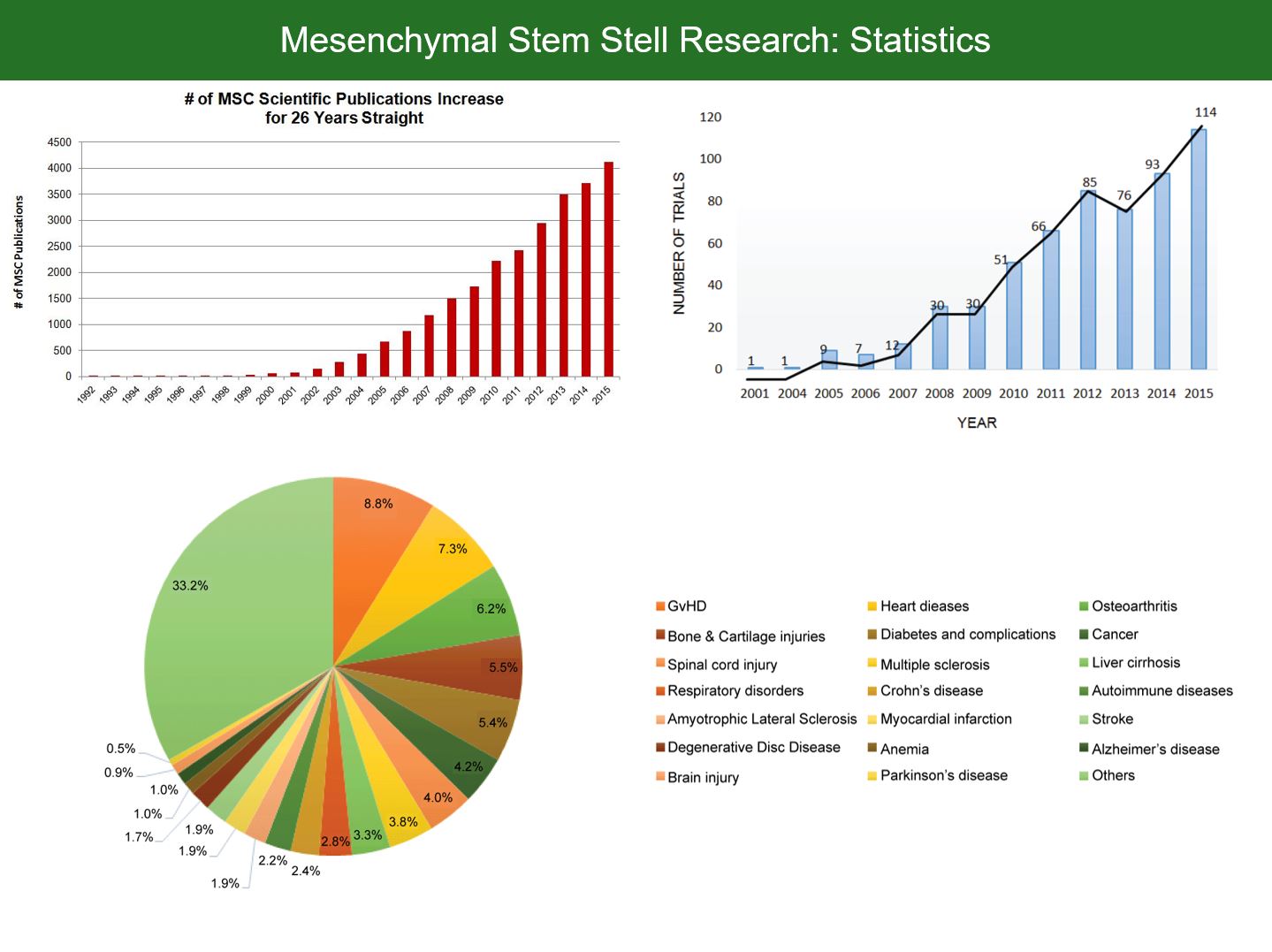
Stem cell research has become an enormous area of research with an exponentially increasing number of original research publications and clinical trials. However, as usual, it takes at least a decade until the latest insights from bio-science are introduced to medical practice. As is the case with stem cell therapies. While the current hot topic of mesenchymal stem cells (MSCs) has shifted towards the question how stem cells actually interact with the surrounding (paracrine/soluble factors, trophic factors, extracellular vesicles, micro-vesicles, exosomes etc.), clinical research has just started with rather crude MSC injection protocols. In clinical practice, though being used at some specialized sites, MSC therapies have not even found broad acceptance in diseases where their safety/efficacy profile is both proven and superior (or even the only known potential therapy). Researchers and progressive thinking medical professionals being frustrated with the slow adaption and evolution of medicine, hence started calling for translational medicine taking a more important place in society.
The ANOVA Institute for Regenerative Medicine is dedicated to just that: A fully equipped medical institution with an own GMP grade lab – dedicated to translational, regenerative cellular medicine. Respecting guideline procedures but not ignoring latest results from stem cell research or other related research fields.
Next Generation Stem Cell Therapy: Why We Do Not “Just Inject Stem Cells” Anymore
ANOVA has been actively using stem cell therapies since 2010. The gap between what was possible in clinical practice and what was known from research was large. Due to a lack of data, regulations and available products, therapies consisted mostly of image guided injections of bone marrow concentrate (BMC) into different compartments with slightly individualized protocols. The results were often astonishing, but the crudeness of this approach was unsatisfying. With increasing knowledge on the pathways stem cells use to make their effects came a paradigm shift of what is possible in clinical practice today. The evolution is best summarized by Zhang et al. in their 2016 review about extracellular vesicles (EVs):
“The intense research focus on stem and progenitor cells could be attributed to their differentiation potential to generate new cells to replace diseased or lost cells in many highly intractable degenerative diseases, such as Alzheimer`s disease, multiple sclerosis, and heart diseases.
However, experimental and clinical studies have increasingly attributed the therapeutic efficacy of these cells to their secretion. While stem and progenitor cells secreted many therapeutic molecules, none of these molecules singly or in combination could recapitulate the functional effects of stem cell transplantations.
Recently, it was reported that extracellular vesicles (EVs) could recapitulate the therapeutic effects of stem cell transplantation. Based on the observations reported thus far, the prevailing hypothesis is that stem cell EVs exert their therapeutic effects by transferring biologically active molecules such as proteins, lipids, mRNA, and microRNA from the stem cells to injured or diseased cells. In this respect, stem cell EVs are similar to EVs from other cell types. They are both primarily vehicles for intercellular communication. Therefore, the differentiating factor is likely due to the composition of their cargo. The cargo of EVs from different cell types are known to include a common set of proteins and also proteins that reflect the cell source of the EVs and the physiological or pathological state of the cell source.”
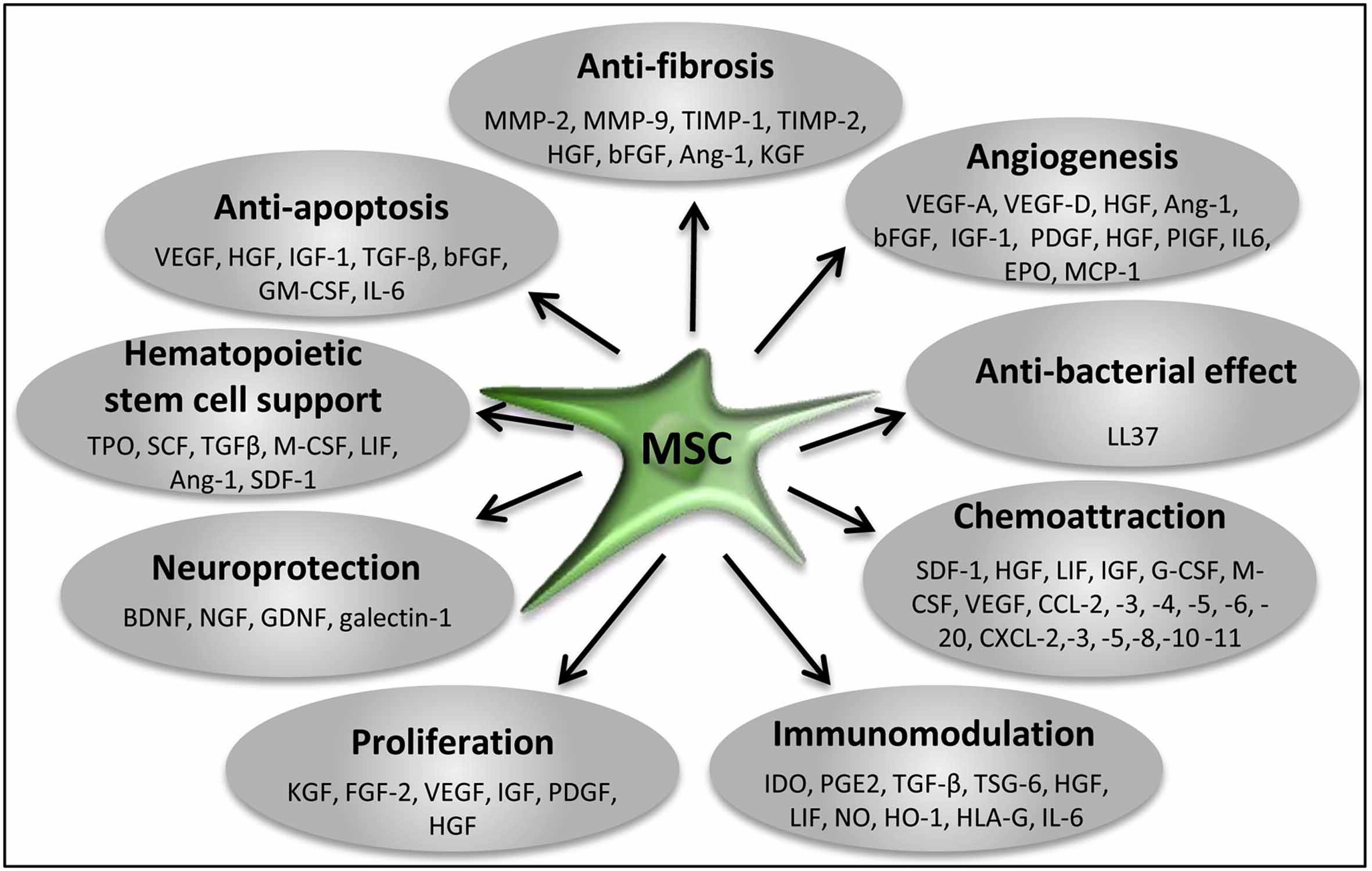
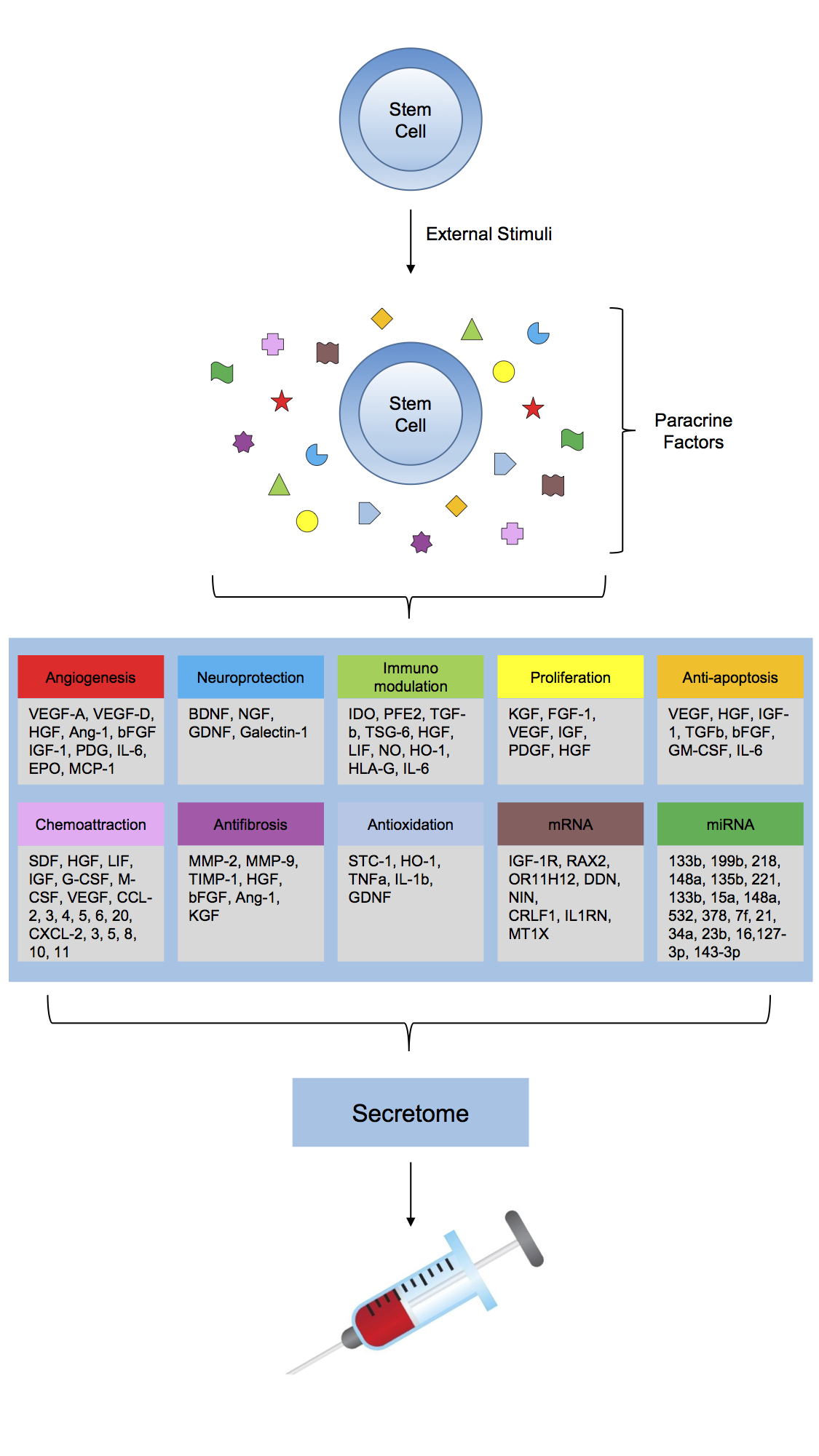
The paracrine activities of stem and progenitor cells are now being extensively explored and mapped. Their major involvement in all essential effects of stem cell therapies (immunomodulation, proliferation, anti-inflammatory, anti-fibrotic, anti-apoptotic, angiogenic, mitogenic, neurotrophic, neuroprotective, chemoattraction) have been shown.
This hence has brought many clinical stem cell groups, us among them, to refocus towards designing new methods with the soluble factors in focus - not the stem cell count. The Stem Cell Secretome Therapy uses MSCs and exposes them to specific stress preconditioning with several proprietary methods to force MSCs to mass produce a secretome with maximum efficacy for the different disease models.
Trophic Factors and Exosome/MV Loads Produced by MSCs and Suggested Functions for Tissue Regeneration/Repair
Angiopoietin: induce angiogenesis, promote cell survival
BDNF: promote neuronal cell survival and differentiation, reduce infarct size
BMPs: regulate tissue homeostasis, promote neurogenesis, induce stromal cell proliferation and migration, promote angiogenesis
CNTF: promote neuronal cell survival
EGF: induce cell proliferation and differentiation
EPO: induce angiogenesis, inhibit apoptosis
FGFs: induce angiogenesis, inhibit apoptosis
Galectins: suppress inflammation, induce stem cell mobilization, inhibit immune cell proliferation
GDNF: promote neuronal cell survival, induce axonal growth, reduce infarct size
G-CSF: induce stem/progenitor cell proliferation, promote neuronal differentiation
HGF: promote progenitor cell mobilization, induce angiogenesis and cell proliferation, inhibit immune cell proliferation
Hemoxygenase-1: promote induction for regulatory T cells
IGFs: induce cell proliferation, inhibit apoptosis
IL-6: stimulate stem/progenitor cell proliferation, induce angiogenesis
IDO: induce regulatory T cells, inhibit T cell activation
IL-1β: suppress inflammation
KGF: induce cell proliferation
MCP-1: induce angiogenesis, induce MSC migration, inhibit apoptosis
MIF: inhibit macrophage migration
NGF: protect neural cells
PDGF: induce cell proliferation
PGE2: suppress inflammation, inhibit immune cell proliferation
SCF: induce stem/progenitor cell proliferation, promote neuronal differentiation
SDF-1: regulate progenitor cell mobilization
TGF-β: induce stem cell differentiation, reduce inflammation/immune activation
TSG-6: suppress immune activation
VEGFs: induce angiogenesis, promote progenitor cell mobilization, inhibit apoptosis
Protein contents of MSC-EVs.
mRNAs expressed in MSC-EVs.
miRNAs expressed in MSC-EVs.
The Stem Cell Secretome: The Essence of Mesenchymal Stem Cell Therapies in a Designed Process
As described, we evolved to design a stem cell secretome using mesenchymal stem cells for our injection therapies. The stem cell secretome begins with adipose tissue derived stem cells being expanded using GMP materials and methods only. Once expended to a suitable amount of vital non-senescent MSCs, a proprietary conditioning process is employed to use the MSCs as small factories to produce massive amounts of their secretome. The amount of secretion is estimated 100-10.000x higher than the same amount of stem cells would secrete when injected. After GMP grade quality controls, this conditioned media is now ready for local or systemic injections. Despite the obvious potency advantage, this brings several further advantages:
- Higher potency by several orders of magnitude
- Hence excellent repeatability of the treatment as a high amount of injection can be produced from one micro liposuction
- Extremely high safety because all major risk factors of MSCs are eliminated:
- local immune responses that can lead long-term rejection of transplanted MSCs
- disruption of local tissue homeostasis causing inflammation
- increased risk of tumor formation due to long-term ex vivo expansion and/or local immunosuppression
- ectopic tissue formation of donor
- Adaptability of the secretome profile to the requirements
References and Literature - Stem Cell Secretome
- Konala, Vijay Bhaskar Reddy, et al. "The current landscape of the mesenchymal stromal cell secretome: a new paradigm for cell-free regeneration." Cytotherapy 18.1 (2016): 13-24.
- Lopez-Verrilli, M. A., et al. "Mesenchymal stem cell-derived exosomes from different sources selectively promote neuritic outgrowth." Neuroscience 320 (2016): 129-139.
- Kim, Hyun Ok, Seong-Mi Choi, and Han-Soo Kim. "Mesenchymal stem cell-derived secretome and microvesicles as a cell-free therapeutics for neurodegenerative disorders." Tissue Engineering and Regenerative Medicine 10.3 (2013): 93-101.
- Rani, Sweta, et al. "Mesenchymal stem cell-derived extracellular vesicles: toward cell-free therapeutic applications." Molecular Therapy 23.5 (2015): 812-823.
- Zhang, Xiaoyan, et al. "Mesenchymal Stem Cell-Derived Extracellular Vesicles: Roles in Tumor Growth, Progression, and Drug Resistance." Stem Cells International 2017 (2017).
- omzikova, Marina O., and Albert A. Rizvanov. "Current Trends in Regenerative Medicine: From Cell to Cell-Free Therapy." BioNanoScience (2016): 1-6.
- Zhang, Bin, et al. "Focus on extracellular vesicles: Therapeutic potential of stem cell-derived extracellular Vesicles." International journal of molecular sciences 17.2 (2016): 174.
- Katsuda T. et al. (2013). Human adipose tissue-derived mesenchymal stem cells secrete functional neprilysin-bound exosomes. Scientific reports, 3, 1197.
- Pusic A. D. et al. (2014). IFNγ-stimulated dendritic cell exosomes as a potential therapeutic for remyelination. Journal of neuroimmunology, 266(1), 12-23.
- Maumus M, Jorgensen C, Noël D (2013) Mesenchymal stem cells in regenerative medicine applied to rheumatic diseases: Role of secretome and exosomes. Biochimie 95:2229–2234. doi: 10.1016/j.biochi.2013.04.017
- Drago D, Cossetti C, Iraci N, et al (2013) Biochimie The stem cell secretome and its role in brain repair. Biochimie 95:2271–2285. doi: 10.1016/j.biochi.2013.06.020
- Sevivas N, Teixeira FG, Portugal R, et al (2016) Mesenchymal Stem Cell Secretome: A Potential Tool for the Prevention of Muscle Degenerative Changes Associated With Chronic Rotator Cuff Tears. Am J Sports Med. doi: 10.1177/0363546516657827
- Hs K (2016) Mesenchymal Stem Cells vs . Mesenchymal Stem Cell Secretome for Rheumatoid Arthritis Treatment. 1:1–2.
- Maumus M, Jorgensen C, Noël D (2013) Mesenchymal stem cells in regenerative medicine applied to rheumatic diseases: Role of secretome and exosomes. Biochimie 95:2229–2234. doi: 10.1016/j.biochi.2013.04.017
- Kapur SK, Katz AJ (2013) Biochimie Review of the adipose derived stem cell secretome. Biochimie 95:2222–2228. doi: 10.1016/j.biochi.2013.06.001
- Ranganath SH, Levy O, Inamdar MS, Karp JM (2012) Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell 10:244–258. doi: 10.1016/j.stem.2012.02.005
- Tran C, Damaser MS (2015) Stem cells as drug delivery methods: Application of stem cell secretome for regeneration. Adv Drug Deliv Rev 82:1–11. doi: 10.1016/j.addr.2014.10.007
- Zimmerlin L, Park TS, Zambidis ET, et al (2013) Mesenchymal stem cell secretome and regenerative therapy after cancer. Biochimie 95:2235–2245. doi: 10.1016/j.biochi.2013.05.010
- Calamia V, Lourido L, Fernandez-Puente P, et al (2012) Secretome analysis of chondroitin sulfate-treated chondrocytes reveals its anti-angiogenic, anti-inflammatory and anti-catabolic properties. Arthritis Res Ther 14:R202. doi: 10.1186/ar4040
- Ranganath SH, Levy O, Inamdar MS, Karp JM (2012) Review Harnessing the Mesenchymal Stem Cell Secretome for the Treatment of Cardiovascular Disease. Stem Cell 10:244–258. doi: 10.1016/j.stem.2012.02.005
- Teixeira FG, Carvalho MM, Sousa N, Salgado AJ (2013) Mesenchymal stem cells secretome: A new paradigm for central nervous system regeneration? Cell Mol Life Sci 70:3871–3882. doi: 10.1007/s00018-013-1290-8
- Kapur SK, Katz AJ (2013) Review of the adipose derived stem cell secretome. Biochimie 95:2222–2228. doi: 10.1016/j.biochi.2013.06.001
- Chang C-P, Chio C-C, Cheong C-U, et al (2013) Hypoxic preconditioning enhances the therapeutic potential of the secretome from cultured human mesenchymal stem cells in experimental traumatic brain injury. Clin Sci (Lond) 124:165–76. doi: 10.1042/CS20120226
- Salgado AJ, Sousa JC, Costa BM, et al (2015) Mesenchymal stem cells secretome as a modulator of the neurogenic niche: basic insights and therapeutic opportunities. Front Cell Neurosci 9:1–18. doi: 10.3389/fncel.2015.00249
- Ahmed NE-MB, Murakami M, Hirose Y, Nakashima M (2016) Therapeutic Potential of Dental Pulp Stem Cell Secretome for Alzheimer’s Disease Treatment: An In Vitro Study. Stem Cells Int 2016:8102478. doi: 10.1155/2016/8102478
- Bhaskar V, Konala R, Mamidi MK, et al (2016) The current landscape of the mesenchymal stromal cell secretome : A new paradigm for cell-free regeneration. Cytotherapy 18:13–24. doi: 10.1016/j.jcyt.2015.10.008
- Malda J, Boere J, van de Lest C, et al (2016) Extracellular vesicles - new tool for joint repair and regeneration - IN PRESS. Nat Rev Rheumatol 12:243–249. doi: 10.1038/nrrheum.2015.170
- Lener T, Gimona M, Aigner L, et al (2015) Applying extracellular vesicles based therapeutics in clinical trials Á an ISEV position paper. 1:1–31.
- Dostert G, Mesure B, Menu P, Velot É (2017) How Do Mesenchymal Stem Cells Influence or Are Influenced by Microenvironment through Extracellular Vesicles Communication ? 5:1–7. doi: 10.3389/fcell.2017.00006
- Joshi P, Benussi L, Furlan R, et al (2015) Extracellular vesicles in Alzheimer’s disease: Friends or foes? focus on Aβ-vesicle interaction. Int. J. Mol. Sci. 16:4800–4813.
- Gao T, Guo W, Chen M, et al (2016) Extracellular Vesicles and Autophagy in Osteoarthritis.
- Katsuda T, Ochiya T (2015) Molecular signatures of mesenchymal stem cell-derived extracellular vesicle-mediated tissue repair. Stem Cell Res Ther 6:212. doi: 10.1186/s13287-015-0214-y
- Lener T, Gioma M, Aigner L, et al (2015) Applying extracellular vesicles based therapeutics in clinical trials - an ISEV position paper. J Extracell Vesicles 4:1–31. doi: 10.3402/jev.v4.30087
- Xu Y, Guo S, Wei C, et al (2016) The Comparison of Adipose Stem Cell and Placental Stem Cell in Secretion Characteristics and in Facial Antiaging.
- Buul GM Van, Villafuertes E, Bos PK, et al (2012) Mesenchymal stem cells secrete factors that inhibit in fl ammatory processes in short-term osteoarthritic synovium and cartilage explant culture. Osteoarthr Cartil 20:1186–1196. doi: 10.1016/j.joca.2012.06.003
- Baglio SR, Pegtel DM, Baldini N (2012) Mesenchymal stem cell secreted vesicles provide novel opportunities in ( stem ) cell-free therapy. 3:1–11. doi: 10.3389/fphys.2012.00359
- Anderson JD, Pham MT, Contreras Z, et al (2016) Mesenchymal stem cell-based therapy for ischemic stroke. Chinese Neurosurg J 2:36. doi: 10.1186/s41016-016-0053-4
- Biology C, Cell R, Eye N, Institutes N (2017) Bone Marrow-Derived Mesenchymal Stem Cells-Derived Exosomes Promote Survival of Retinal Ganglion Cells Through miRNA-Dependent Mechanisms. 1273–1285.
References and Literature - Mesenchymal Stem Cells 'MSCs'
- Gu W, Zhang F, Xue Q, Ma Z, Lu P, Yu B. Transplantation of bone marrow mesenchymal stem cells reduces lesion volume and induces axonal regrowth of injured spinal cord. Neuropathology. 2010; 30: 205-217.
- Wilkins A, Kemp K, Ginty M, Hares K, Mallam E, Scolding N. Human bone marrow-derived mesenchymal stem cells secrete brain-derived neurotrophic factor which promotes neuronal survival in vitro. Stem Cell Res. 2009; 3: 6370
- Wei X, Yang X, Han ZP, Qu FF, Shao L, Shi YF. Mesenchymal stem cells: a new trend for cell therapy. Acta Pharmacol Sin. 2013; 34: 747-754.
- Wang S, Qu X, Zhao RC. Clinical applications of mesenchymal stem cells. J Hematol Oncol. 2014; 5: 19.
- Farini, Andrea, et al. "Clinical applications of mesenchymal stem cells in chronic diseases." Stem cells international 2014 (2014).
- Volarevic, Vladislav, et al. "Concise review: therapeutic potential of mesenchymal stem cells for the treatment of acute liver failure and cirrhosis." Stem Cells 32.11 (2014): 2818-2823.
- Ikebe, Chiho, and Ken Suzuki. "Mesenchymal stem cells for regenerative therapy: optimization of cell preparation protocols." BioMed research international 2014 (2014).
- Sharma, Ratti Ram, et al. "Mesenchymal stem or stromal cells: a review of clinical applications and manufacturing practices." Transfusion 54.5 (2014): 1418-1437.
- Jo, Chris Hyunchul, et al. "Intra‐articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof‐of‐concept clinical trial." Stem cells 32.5 (2014): 1254-1266.
- Squillaro, Tiziana, Gianfranco Peluso, and Umberto Galderisi. "Clinical trials with mesenchymal stem cells: an update." Cell transplantation 25.5 (2016): 829-848.
- Orozco, Lluis, et al. "Treatment of knee osteoarthritis with autologous mesenchymal stem cells: two-year follow-up results." Transplantation 97.11 (2014): e66-e68.
- Filardo, Giuseppe, et al. "Mesenchymal stem cells for the treatment of cartilage lesions: from preclinical findings to clinical application in orthopaedics." Knee surgery, sports traumatology, arthroscopy 21.8 (2013): 1717-1729.
- Jo, Chris Hyunchul, et al. "Intra‐articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof‐of‐concept clinical trial." Stem cells 32.5 (2014): 1254-1266.
- Vangsness, C. Thomas, et al. "Adult human mesenchymal stem cells delivered via intra-articular injection to the knee following partial medial meniscectomy." J Bone Joint Surg Am 96.2 (2014): 90-98.
References and Literature - Bone Marrow Concentrate 'BMC'
- He Y, He W, Qin G, Luo J, Xiao M. Transplantation KCNMA1 modified bone marrow-mesenchymal stem cell therapy for diabetes mellitus-induced erectile dysfunction. Andrologia. 2014;46(5):479-486. doi:10.1111/and.12104.
- Mathiasen AB, Qayyum AA, Jørgensen E, et al. Bone marrow-derived mesenchymal stromal cell treatment in patients with severe ischaemic heart failure: a randomized placebo-controlled trial ({MSC}-{HF} trial). Eur Heart J. 2015;36(27):1744-1753. doi:10.1093/eurheartj/ehv136.
- Mathiasen AB, Qayyum AA, Jørgensen E, et al. Interventional cardiology Bone marrow-derived mesenchymal stromal cell treatment in patients with severe ischaemic heart failure?: a randomized placebo-controlled trial. 2015. doi:10.1093/eurheartj/ehv136.
- Liao H-T, Chen C-T. Osteogenic potential: Comparison between bone marrow and adipose-derived mesenchymal stem cells. World J Stem Cells. 2014;6(3):288-295. doi:10.4252/wjsc.v6.i3.288.
- Terai S, Ishikawa T, Omori K, et al. Improved liver function in patients with liver cirrhosis after autologous bone marrow cell infusion therapy. Stem Cells. 2006;24(10):2292-2298. doi:10.1634/stemcells.2005-0542.
- 2015_Cao_Spine-Journal_Bone-marrow-mesenchymal-stem-cells-slow-intervertebral-disc-degeneration-through-the-NF-?B-pathway.pdf.
- Zhao J, Zhang Q, Wang Y, Li Y. Uterine Infusion With Bone Marrow Mesenchymal Stem Cells Improves Endometrium Thickness in a Rat Model of Thin Endometrium. Reprod Sci. 2015;22(2):181-188. doi:10.1177/1933719114537715.
- Fekete N, Rojewski MT, Fürst D, et al. GMP-compliant isolation and large-scale expansion of bone marrow-derived MSC. PLoS One. 2012;7(8). doi:10.1371/journal.pone.0043255.
- Elman JS, Li M, Wang F, Gimble JM, Parekkadan B. A comparison of adipose and bone marrow-derived mesenchymal stromal cell secreted factors in the treatment of systemic inflammation. 2014:4-11.
- Li C, Wu X, Tong J, et al. Comparative analysis of human mesenchymal stem cells from bone marrow and adipose tissue under xeno-free conditions for cell therapy. Stem Cell Res Ther. 2015;6(1):55. doi:10.1186/s13287-015-0066-5.
- Books J, Sign R. Safety of Intracavernous Bone Marrow-Mononuclear Cells for Postrad ... Safety of Intracavernous Bone Marrow-Mononuclear Cells for Postradical Prostatectomy Erectile Dysfunction?: An Open Dose-Escalation Pilot Study Safety of Intracavernous Bone Marrow-Mon. 2017:2015-2017.
- Rinker TE, Hammoudi TM, Kemp ML, Lu H, Temenoff JS. Interactions between mesenchymal stem cells, adipocytes, and osteoblasts in a 3D tri-culture model of hyperglycemic conditions in the bone marrow microenvironment. Integr Biol (Camb). 2014;6(3):324-337. doi:10.1039/c3ib40194d.
- Al-sayegh H, Bashir J, Goodyear S, Freeman MD. A prospective multi-site registry study of a specific protocol of autologous bone marrow concentrate for the treatment of shoulder rotator cuff tears and osteoarthritis. 2015:269-276.
- Rager TM, Olson JK, Zhou Y, Wang Y, Besner GE. Exosomes secreted from bone marrow-derived mesenchymal stem cells protect the intestines from experimental necrotizing enterocolitis. J Pediatr Surg. 2016;51(6):942-947. doi:10.1016/j.jpedsurg.2016.02.061.
- Tate-oliver K, Alexander RW. Density Platelet-Rich Plasma or Bone Marrow.
- Tang K, Yan J, Shen Y, et al. Tracing type 1 diabetic Tibet miniature pig ? s bone marrow mesenchymal stem cells in vitro by magnetic resonance imaging. 2014;6:123-131. doi:10.1111/1753-0407.12084.
- Wang X, Mamillapalli R, Mutlu L, Du H, Taylor HS. Chemoattraction of bone marrow-derived stem cells towards human endometrial stromal cells is mediated by estradiol regulated CXCL12 and CXCR4 expression. Stem Cell Res. 2015;15(1):14-22. doi:10.1016/j.scr.2015.04.004.
- Rambaldi A, Capelli C, Domenghini M, et al. Human platelet lysate allows expansion and clinical grade production of mesenchymal stromal cells from small samples of bone marrow aspirates or marrow filter washouts. 2007:785-791. doi:10.1038/sj.bmt.1705798.
- Mushtaq M, Williams AR, Suncion VY, et al. Transendocardial Mesenchymal Stem Cells and Mononuclear Bone Marrow Cells for Ischemic Cardiomyopathy The TAC-HFT Randomized Trial. 2015;16960. doi:10.1001/jama.2013.282909.
- 2014 Autografting of bone marrow mesenchymal stem cells alleviates streptozotocin induced diabetes in miniature pigs.pdf.
- Program T, Marga- P, Kingdom U. Bone Marrow Therapies for Chronic Heart Dis- ease. 2015:1-12. doi:10.1002/stem.2080.
- Biology C, Cell R, Eye N, Institutes N, Infor- AS. Bone Marrow-Derived Mesenchymal Stem Cells-Derived Exosomes Promote Survival of Retinal Ganglion Cells Through miRNA-Dependent Mechanisms. 2017:1273-1285. doi:10.1002/sctm.12056.
- Jeong Y, Kyu H, Hwa H, Chan Y. Cellular Physiology and Biochemistr y Biochemistry Direct Comparison of Human Mesenchymal Stem Cells Derived from Adipose Tissues and Bone Marrow in Mediating Neovascularization in Response to Vascular Ischemia. Cell Physiol Biochem. 2007;20:867-876.
- Shutian S, Shaoping N, Xingxin W, et al. GW25-e3198 The combination of transforming growth factor ?1 and 5-azacytidine improve the differentiation effects of rat Bone marrow mesenchymal stem cells into cardiomyocytes. J Am Coll Cardiol. 2014;64(16):C21. doi:10.1016/j.jacc.2014.06.105.
- Narita T, Suzuki K. Bone marrow-derived mesenchymal stem cells for the treatment of heart failure. Heart Fail Rev. 2014:53-68. doi:10.1007/s10741-014-9435-x.
- Elman JS, Li M, Wang F, Gimble JM, Parekkadan B. A comparison of adipose and bone marrow-derived mesenchymal stromal cell secreted factors in the treatment of systemic inflammation. J Inflamm (Lond). 2014;11:1. doi:10.1186/1476-9255-11-1.
- Dong X, Zhu F, Liu Q, et al. Transplanted bone marrow mesenchymal stem cells protects myocardium by regulating 14-3-3 protein in a rat model of diabetic cardiomyopathy. 2014;7(7):3714-3723.
- Huang L, Wu W, Luo F. Umbilical Cord Mesenchymal Stromal Cell With Autologous Bone Marrow Cell Transplantation in Established Type 1 Diabetes?: A Pilot Randomized Controlled Open-Label Clinical Study to Assess Safety and Impact on Insulin Secretion. 2016:1-9. doi:10.2337/dc15-0171.
- Sanghi V, Sethi D, Harris KL, et al. International Journal of the Cardiovascular Academy Autologous bone marrow concentrate enriched in progenitor cells ? An adjuvant in the treatment of acute myocardial infarction. IJCAC. 2016. doi:10.1016/j.ijcac.2016.04.001.
- Leyh M, Seitz A, Dürselen L, et al. Subchondral bone influences chondrogenic differentiation and collagen production of human bone marrow-derived mesenchymal stem cells and articular chondrocytes. 2014:1-18. doi:10.1186/s13075-014-0453-9.
- Surgery M, Stomatological S, Material CP, et al. T ISSUE -S PECIFIC S TEM C ELLS Adiponectin Regulates Bone Marrow Mesenchymal Stem Cell Niche Through a Unique Signal Transduction Pathway?: An Approach for Treating Bone Disease in Diabetes. 2015:240-252.
- Li C, Wu X, Tong J, et al. Comparative analysis of human mesenchymal stem cells from bone marrow and adipose tissue under xeno-free conditions for cell therapy. Stem Cell Res Ther. 2015;6(1):55. doi:10.1186/s13287-015-0066-5.
- Mead B, Logan A, Berry M, Leadbeater W, Scheven BA. Paracrine-Mediated Neuroprotection and Neuritogenesis of Axotomised Retinal Ganglion Cells by Human Dental Pulp Stem Cells: Comparison with Human Bone Marrow and Adipose-Derived Mesenchymal Stem Cells. PLoS One. 2014;9(10):e109305. doi:10.1371/journal.pone.0109305.
- Cao C, Zou J, Liu X, Shapiro A. Bone marrow mesenchymal stem cells slow intervertebral disc degeneration through the NF- k B pathway. Spine J. 2015;15(3):530-538. doi:10.1016/j.spinee.2014.11.021.
- Li C, Wu X, Tong J, et al. Comparative analysis of human mesenchymal stem cells from bone marrow and adipose tissue under xeno-free conditions for cell therapy. ??? 2015. doi:10.1186/s13287-015-0066-5.
- Narita T, Suzuki K. Bone marrow-derived mesenchymal stem cells for the treatment of heart failure. 2015:53-68. doi:10.1007/s10741-014-9435-x.
- Heldman AW, DiFede DL, Fishman JE, et al. Transendocardial Mesenchymal Stem Cells and Mononuclear Bone Marrow Cells for Ischemic Cardiomyopathy. Jama. 2014;311(1):62. doi:10.1001/jama.2013.282909.
- Baglio SR, Rooijers K, Koppers-Lalic D, et al. Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res Ther. 2015;6(1):127. doi:10.1186/s13287-015-0116-z.
- Islam MN, Das SR, Emin MT, et al. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med. 2012;18(5):759-765. doi:10.1038/nm.2736.
- Baglio SR, Rooijers K, Koppers-Lalic D, et al. Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res Ther. 2015;6(1):127. doi:10.1186/s13287-015-0116-z.
- Bian S, Zhang L, Duan L, et al. Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model. J Mol Med (Berl). 2014;92(4):387-397. doi:10.1007/s00109-013-1110-5.
- Shabbir A, Zisa D, Suzuki G, Lee T. Heart failure therapy mediated by the trophic activities of bone marrow mesenchymal stem cells: a noninvasive therapeutic regimen. Am J Physiol Heart Circ Physiol. 2009;296(6):H1888-H1897. doi:10.1152/ajpheart.00186.2009.
- Czubak PB, Bojarska-junak A, Tabarkiewicz J, Putowski LB. A Modified Method of Insulin Producing Cells ? Generation from Bone Marrow-Derived Mesenchymal Stem Cells. 2014;2014:1-7. doi:10.1155/2014/628591.
- Baglio SR, Rooijers K, Koppers-Lalic D, et al. Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res Ther. 2015;6(1):127. doi:10.1186/s13287-015-0116-z.
- am Esch JS, Knoefel WT, Klein M, et al. Portal application of autologous CD133+ bone marrow cells to the liver: a novel concept to support hepatic regeneration. Stem Cells. 2005;23(4):463-470. doi:10.1634/stemcells.2004-0283.
- Wang X, Nie S-P, Zhen L, et al. TCTAP A-156 Retrograde Coronary Vein Delivery of Basic Fibroblast Growth Enhances Bone Marrow Mesenchymal Stem Cells Engraftment for Myocardial Repair in a Canine Infarct Model. J Am Coll Cardiol. 2014;63(12):S44. doi:10.1016/j.jacc.2014.02.189.
- Al-sayegh H, Bashir J, Goodyear S, Freeman MD. A prospective multi-site registry study of a specific protocol of autologous bone marrow concentrate for the treatment of shoulder rotator cuff tears and osteoarthritis. 2015:269-276.
- Gabr MM, Zakaria MM, Refaie AF, et al. Differentiation of Human Bone Marrow-Derived Mesenchymal Stem Cells into Insulin-Producing Cells?: Evidence for Further Maturation In Vivo. 2015;2015.
- Pai M, Zacharoulis D, Milicevic MN, et al. Autologous infusion of expanded mobilized adult bone marrow-derived CD34+ cells into patients with alcoholic liver cirrhosis. Am J Gastroenterol. 2008;103(8):1952-1958. doi:10.1111/j.1572-0241.2008.01993.x.
- Kushida T, Iida H. Bone marrow cell transplantation efficiently repairs tendon and ligament injuries. Front Cell Dev Biol. 2014;2(July):1-4. doi:10.3389/fcell.2014.00027.
- Schulte J, Knoefel T, Klein M, et al. Portal Application of Autologous CD133 + Bone Marrow Cells to the. 2005:463-470. doi:10.1634/stemcells.2004-0283.
- Scott M, Ph D, Marley SB, et al. Autologous Infusion of Expanded Mobilized Adult Bone Marrow-Derived CD34 + Cells Into Patients With Alcoholic Liver Cirrhosis. 2008:1952-1958. doi:10.1111/j.1572-0241.2008.01993.x.
- 2015 Murine Sca1+Lin? bone marrow contains an endodermal precursor population that differentiates into hepatocytes.pdf.
- Prabhakar S, Marwaha N, Lal V, Sharma RR, Rajan R, Khandelwal N. Autologous bone marrow-derived stem cells in amyotrophic lateral sclerosis: A pilot study. Neurol India. 2012;60(5):465-469. doi:10.4103/0028-3886.103185.
- Naaldijk Y, Jäger C, Fabian C, et al. Effect of systemic transplantation of bone marrow-derived mesenchymal stem cells on neuropathology markers in APP/PS1 Alzheimer mice. Neuropathol Appl Neurobiol. 2016:1-16. doi:10.1111/nan.12319.
- Nicola M Di, Carlo-stella C, Magni M, et al. induced by cellular or nonspecific mitogenic stimuli Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. 2013;99(10):3838-3843. doi:10.1182/blood.V99.10.3838.
- Tzameret A, Sher I, Belkin M, et al. Epiretinal transplantation of human bone marrow mesenchymal stem cells rescues retinal and vision function in a rat model of retinal degeneration. Stem Cell Res. 2015;15(2):387-394. doi:10.1016/j.scr.2015.08.007.
- Augello A, Tasso R, Negrini SM, Cancedda R, Pennesi G. Cell therapy using allogeneic bone marrow mesenchymal stem cells prevents tissue damage in collagen-induced arthritis. Arthritis Rheum. 2007;56(4):1175-1186. doi:10.1002/art.22511.
- Capelli C, Domenghini M, Borleri G, et al. Human platelet lysate allows expansion and clinical grade production of mesenchymal stromal cells from small samples of bone marrow aspirates or marrow filter washouts. Bone Marrow Transplant. 2007;40(8):785-791. doi:10.1038/sj.bmt.1705798.
- Sanghi V, Sethi D, Harris KL, et al. International Journal of the Cardiovascular Academy Autologous bone marrow concentrate enriched in progenitor cells ? An adjuvant in the treatment of acute myocardial infarction. IJCAC. 2016. doi:10.1016/j.ijcac.2016.04.001.
- Oe K, Kushida T, Okamoto N, et al. New strategies for anterior cruciate ligament partial rupture using bone marrow transplantation in rats. Stem Cells Dev. 2011;20(4):671-679. doi:10.1089/scd.2010.0182.
- Ahmed HH, Salem AM, Atta HM, et al. Updates in the pathophysiological mechanisms of Parkinson?s disease: Emerging role of bone marrow mesenchymal stem cells. World J Stem Cells. 2016;8(3):106. doi:10.4252/wjsc.v8.i3.106.
- Cai J, Wu Z, Xu X, et al. Umbilical Cord Mesenchymal Stromal Cell With Autologous Bone Marrow Cell Transplantation in Established Type 1 Diabetes: A Pilot Randomized Controlled Open-Label Clinical Study to Assess Safety and Impact on Insulin Secretion. Diabetes Care. 2015:dc150171. doi:10.2337/dc15-0171.
- Abdel Aziz MT, Wassef MAA, Ahmed HH, et al. The role of bone marrow derived-mesenchymal stem cells in attenuation of kidney function in rats with diabetic nephropathy. Diabetol Metab Syndr. 2014;6(1):34. doi:10.1186/1758-5996-6-34.
- Al-sayegh H, Bashir J, Goodyear S, Freeman MD. A prospective multi-site registry study of a specific protocol of autologous bone marrow concentrate for the treatment of shoulder rotator cuff tears and osteoarthritis. 2015:269-276.
- Shabbir A, Zisa D, Suzuki G, Lee T. Heart failure therapy mediated by the trophic activities of bone marrow mesenchymal stem cells: a noninvasive therapeutic regimen. Am J Physiol Heart Circ Physiol. 2009;296(6):H1888-H1897. doi:10.1152/ajpheart.00186.2009.
- Collino F, Deregibus MC, Bruno S, et al. Microvesicles derived from adult human bone marrow and tissue specific mesenchymal stem cells shuttle selected pattern of miRNAs. PLoS One. 2010;5(7). doi:10.1371/journal.pone.0011803.
- Sanghi V, Sethi D, Harris KL, et al. International Journal of the Cardiovascular Academy Autologous bone marrow concentrate enriched in progenitor cells ? An adjuvant in the treatment of acute myocardial infarction. IJCAC. 2016. doi:10.1016/j.ijcac.2016.04.001.
- Fekete N, Rojewski MT, Fürst D, et al. GMP-compliant isolation and large-scale expansion of bone marrow-derived MSC. PLoS One. 2012;7(8). doi:10.1371/journal.pone.0043255.
- C. L, S.A. M, M. A, S.H. V, A. F-G. Exosomes mediate the cytoprotective effects of bone Marrow-Derived Stromal Cells (MSCS) on the hypoxic lung. Am J Respir Crit Care Med. 2011;183(1 MeetingAbstracts):no pagination. http://ajrccm.atsjournals.org/cgi/reprint/183/1_MeetingAbstracts/A3764?sid=f0b58cd0-9f08-401b-bb88-e9a3268d044f%5Cnhttp://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=emed10&NEWS=N&AN=70848115.
- Islam MN, Das SR, Emin MT, et al. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med. 2012;18(5):759-765. doi:10.1038/nm.2736.
- Yoon SH, Shim YS, Park YH, et al. Complete spinal cord injury treatment using autologous bone marrow cell transplantation and bone marrow stimulation with granulocyte macrophage-colony stimulating factor: Phase I/II clinical trial. Stem Cells. 2007;25(8):2066-2073. doi:10.1634/stemcells.2006-0807.
- Hernigou P, Guissou I, Homma Y, et al. Percutaneous injection of bone marrow mesenchymal stem cells for ankle non-unions decreases complications in patients with diabetes. Int Orthop. 2015. doi:10.1007/s00264-015-2738-2.
- Davies OG, Smith AJ, Cooper PR, Shelton RM, Scheven BA. Cryobiology The effects of cryopreservation on cells isolated from adipose , bone marrow and dental pulp tissues q. Cryobiology. 2014;69(2):342-347. doi:10.1016/j.cryobiol.2014.08.003.
- C. L, S.A. M, M. A, S.H. V, A. F-G. Exosomes mediate the cytoprotective effects of bone Marrow-Derived Stromal Cells (MSCS) on the hypoxic lung. Am J Respir Crit Care Med. 2011;183(1 MeetingAbstracts):no pagination. http://ajrccm.atsjournals.org/cgi/reprint/183/1_MeetingAbstracts/A3764?sid=f0b58cd0-9f08-401b-bb88-e9a3268d044f%5Cnhttp://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=emed10&NEWS=N&AN=70848115.
- Tang KX, Shen YF, Li BY, et al. Tracing type 1 diabetic Tibet miniature pig?s bone marrow mesenchymal stem cells in vitro by magnetic resonance imaging. J Diabetes. 2013;6:123-131. doi:10.1111/1753-0407.12084.
- Associates RM, Biosciences C. T RANSLATIONAL AND C LINICAL Percutaneous Injection of Autologous Bone Marrow Concentrate Cells Significantly Reduces Lumbar Discogenic Pain Through 12 Months. 2015:146-156.
- Kasahara Y, Matsuyama T, Taguchi A. Treatment of Autologous Bone Marrow Mononuclear Cells for Acute and Subacute Stroke Cell Therapy for Acute / Subacute Stroke. 2015:37-46. doi:10.1007/978.
- Qi X, Zhang J, Yuan H, et al. Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells repair critical-sized bone defects through enhanced angiogenesis and osteogenesis in osteoporotic rats. Int J Biol Sci. 2016;12(7):836-849. doi:10.7150/ijbs.14809.
- Shabbir A, Zisa D, Suzuki G, Lee T. Heart failure therapy mediated by the trophic activities of bone marrow mesenchymal stem cells: a noninvasive therapeutic regimen. Am J Physiol Heart Circ Physiol. 2009;296(6):H1888-H1897. doi:10.1152/ajpheart.00186.2009.
- Nicola M Di, Carlo-stella C, Magni M, et al. induced by cellular or nonspecific mitogenic stimuli Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. 2013;99(10):3838-3843. doi:10.1182/blood.V99.10.3838.
- Capelli C, Domenghini M, Borleri G, et al. Human platelet lysate allows expansion and clinical grade production of mesenchymal stromal cells from small samples of bone marrow aspirates or marrow filter washouts. Bone Marrow Transplant. 2007;40(8):785-791. doi:10.1038/sj.bmt.1705798.
- Yiou R, Hamidou L, Birebent B, et al. Safety of Intracavernous Bone Marrow-Mononuclear Cells for Postradical Prostatectomy Erectile Dysfunction: An Open Dose-Escalation Pilot Study. Eur Urol. 2016;69(6):988-991. doi:10.1016/j.eururo.2015.09.026.
- Neural I, Medicine R, Science M. Complete Spinal Cord Injury Treatment Using Autologous Bone Marrow Cell Transplantation and Bone Marrow Stimulation with Granulocyte Macrophage-Colony Stimulating Factor?: Phase I / II Clinical Trial. 2007:2066-2073. doi:10.1634/stemcells.2006-0807.
- Collino F, Deregibus MC, Bruno S, et al. Microvesicles derived from adult human bone marrow and tissue specific mesenchymal stem cells shuttle selected pattern of miRNAs. PLoS One. 2010;5(7). doi:10.1371/journal.pone.0011803.
- Yu L, Tu Q, Han Q, et al. Adiponectin Regulates Bone Marrow Mesenchymal Stem Cell Niche Through a Unique Signal Transduction Pathway?: An Approach for Treating Bone Disease in Diabetes. Stem Cells. 2015;33:240-252. doi:10.1002/stem.1844.
- Lyra AC, Soares MB, da Silva LF, et al. Feasibility and safety of autologous bone marrow mononuclear cell transplantation in patients with advanced chronic liver disease. World J Gastroenterol. 2007;13(7):1067-1073. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17373741.
- Song F, Tang J, Geng R, et al. Comparison of the efficacy of bone marrow mononuclear cells and bone mesenchymal stem cells in the treatment of osteoarthritis in a sheep model. 2014;7(4):1415-1426.
- Nakamura-ishizu A, Takubo K, Kobayashi H, Suzuki-inoue K. CLEC-2 in megakaryocytes is critical for maintenance of hematopoietic stem cells in the bone marrow. 2015. doi:10.1084/jem.20150057.
- Leyh M, Seitz A, Dürselen L, et al. Subchondral bone influences chondrogenic differentiation and collagen production of human bone marrow-derived mesenchymal stem cells and articular chondrocytes. 2014:1-18. doi:10.1186/s13075-014-0453-9.
- Beane OS, Fonseca VC, Cooper LL, Koren G. Impact of Aging on the Regenerative Properties of Bone Marrow- , Muscle- , and Adipose-Derived Mesenchymal Stem / Stromal Cells. 2014:1-22. doi:10.1371/journal.pone.0115963.
- Nicola M Di, Carlo-stella C, Magni M, et al. induced by cellular or nonspecific mitogenic stimuli Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. 2013;99(10):3838-3843. doi:10.1182/blood.V99.10.3838.
- Ribeiro A, Laranjeira P, Mendes S, et al. Mesenchymal stem cells from umbilical cord matrix, adipose tissue and bone marrow exhibit different capability to suppress peripheral blood B, natural killer and T cells. Stem Cell Res Ther. 2013;4(5):125. doi:10.1186/scrt336.
- Observer C, Alto P, Program T, Hospital M. T RANSLATIONAL AND C LINICAL Bone Marrow Therapies for Chronic Heart Disease. 2015:3212-3227. doi:10.1002/stem.2080.
- Beane OS, Fonseca VC, Cooper LL, Koren G. Impact of Aging on the Regenerative Properties of Bone Marrow- , Muscle- , and Adipose-Derived Mesenchymal Stem / Stromal Cells. 2014:1-22. doi:10.1371/journal.pone.0115963.
- Rodriguez-Menocal L, Shareef S, Salgado M, Shabbir A, Van Badiavas E. Role of whole bone marrow, whole bone marrow cultured cells, and mesenchymal stem cells in chronic wound healing. Stem Cell Res Ther. 2015;6(1):1-11. doi:10.1186/s13287-015-0001-9.
- Islam MN, Das SR, Emin MT, et al. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med. 2012;18(5):759-765. doi:10.1038/nm.2736.
- Venkataramana NK, Kumar SK V, Balaraju S, et al. Open-labeled study of unilateral autologous bone-marrow-derived mesenchymal stem cell transplantation in Parkinson?s disease. Transl Res. 2010;155(2):62-70. doi:10.1016/j.trsl.2009.07.006.
- Czubak P, Bojarska-Junak A, Tabarkiewicz J, Putowski L. A modified method of insulin producing cells? generation from bone marrow-derived mesenchymal stem cells. J Diabetes Res. 2014;2014:628591. doi:10.1155/2014/628591.
- Shutian S, Shaoping N, Xingxin W, et al. GW25-e3198 The combination of transforming growth factor ?1 and 5-azacytidine improve the differentiation effects of rat Bone marrow mesenchymal stem cells into cardiomyocytes. J Am Coll Cardiol. 2014;64(16):C21. doi:10.1016/j.jacc.2014.06.105.
- Yang J, Kaur K, Ong LL, Eisenberg CA, Eisenberg LM. Inhibition of G9a Histone Methyltransferase Converts Bone Marrow Mesenchymal Stem Cells to Cardiac Competent Progenitors. Stem Cells Int. 2015;2015:1-12. doi:10.1155/2015/270428.
- Biology C, Cell R, Eye N, Institutes N, Infor- AS. Bone Marrow-Derived Mesenchymal Stem Cells-Derived Exosomes Promote Survival of Retinal Ganglion Cells Through miRNA-Dependent Mechanisms. 2017. doi:10.1002/sctm.12056.
- Chen J, Venkat P, Chopp M. Bone Marrow Mesenchymal Stromal Cell Transplantation?: A Neurorestorative Therapy for Stroke. 2015:47-69. doi:10.1007/978.
- Kondo M, Kamiya H, Himeno T, et al. Therapeutic efficacy of bone marrow-derived mononuclear cells in diabetic polyneuropathy is impaired with aging or diabetes. J Diabetes Investig. 2015;6(2):140-149. doi:10.1111/jdi.12272.
- Nakamura-ishizu A, Takubo K, Kobayashi H, Suzuki-inoue K. CLEC-2 in megakaryocytes is critical for maintenance of hematopoietic stem cells in the bone marrow. 2015. doi:10.1084/jem.20150057.
- Gabr MM, Zakaria MM, Refaie AF, et al. Generation of insulin-producing cells from human bone marrow-derived mesenchymal stem cells: comparison of three differentiation protocols. Biomed Res Int. 2014;2014:832736. doi:10.1155/2014/832736.
- Tzameret A, Sher I, Belkin M, et al. Epiretinal transplantation of human bone marrow mesenchymal stem cells rescues retinal and vision function in a rat model of retinal degeneration ?. Stem Cell Res. 2015;15(2):387-394. doi:10.1016/j.scr.2015.08.007.
- C. L, S.A. M, M. A, S.H. V, A. F-G. Exosomes mediate the cytoprotective effects of bone Marrow-Derived Stromal Cells (MSCS) on the hypoxic lung. Am J Respir Crit Care Med. 2011;183(1 MeetingAbstracts):no pagination. http://ajrccm.atsjournals.org/cgi/reprint/183/1_MeetingAbstracts/A3764?sid=f0b58cd0-9f08-401b-bb88-e9a3268d044f%5Cnhttp://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=emed10&NEWS=N&AN=70848115.
- Sun J, Wei ZZ, Gu X, et al. Intranasal delivery of hypoxia-preconditioned bone marrow-derived mesenchymal stem cells enhanced regenerative effects after intracerebral hemorrhagic stroke in mice. Exp Neurol. 2015. doi:10.1016/j.expneurol.2015.03.011.
- Talaat M, Aziz A, Abdel M, et al. The role of bone marrow derived-mesenchymal stem cells in attenuation of kidney function in rats with diabetic nephropathy. 2014:1-10.
- Song F, Tang J, Geng R, et al. Comparison of the efficacy of bone marrow mononuclear cells and bone mesenchymal stem cells in the treatment of osteoarthritis in a sheep model. 2014;7(4):1415-1426.
- Kallis YN, Alison MR, Forbes SJ. Bone marrow stem cells and liver disease. Gut. 2007;56(5):716-724. doi:10.1136/gut.2006.098442.
- Kondo M, Kamiya H, Himeno T, et al. Therapeutic ef fi cacy of bone marrow-derived mononuclear cells in diabetic polyneuropathy is impaired with aging or diabetes. 2015;6(2). doi:10.1111/jdi.12272.
- Czubak P, Bojarska-Junak A, Tabarkiewicz J, Putowski L. A modified method of insulin producing cells? generation from bone marrow-derived mesenchymal stem cells. J Diabetes Res. 2014;2014:628591. doi:10.1155/2014/628591.
- Beane OS, Fonseca VC, Cooper LL, Koren G. Impact of Aging on the Regenerative Properties of Bone Marrow- , Muscle- , and Adipose-Derived Mesenchymal Stem / Stromal Cells. 2014:1-22. doi:10.1371/journal.pone.0115963.
- Song F, Tang J, Geng R, et al. Comparison of the efficacy of bone marrow mononuclear cells and bone mesenchymal stem cells in the treatment of osteoarthritis in a sheep model. 2014;7(4):1415-1426.
- Davies OG, Smith AJ, Cooper PR, Shelton RM, Scheven BA. Cryobiology The effects of cryopreservation on cells isolated from adipose , bone marrow and dental pulp tissues q. Cryobiology. 2014;69(2):342-347. doi:10.1016/j.cryobiol.2014.08.003.
- Sanz-Ruiz R, Garcia AN, Elizaga J, Fdez-Aviles F. TCT-150 First-in-man Experience with Transendocardial Injections of Bone Marrow-Derived Mesenchymal Stem Cells in Idiopathic Dilated Cardiomyopathy. The MYOCYTE trial. J Am Coll Cardiol. 2014;64(11):B45. doi:10.1016/j.jacc.2014.07.186.
- Totey SM. Open-labeled study of unilateral autologous bone-marrow-derived mesenchymal stem cell transplantation in Parkinsons disease. Transl Res. 2010;155(2):62-70. doi:10.1016/j.trsl.2009.07.006.
- Biology C, Cell R, Eye N, Institutes N. Bone Marrow-Derived Mesenchymal Stem Cells-Derived Exosomes Promote Survival of Retinal Ganglion Cells Through miRNA-Dependent Mechanisms. 2017:1273-1285.
- Biology C, Cell R, Eye N, Institutes N. Bone Marrow-Derived Mesenchymal Stem Cells-Derived Exosomes Promote Survival of Retinal Ganglion Cells Through miRNA-Dependent Mechanisms. 2017.
- Naaldijk Y, J C. Effect of systemic transplantation of bone marrow-derived mesenchymal stem cells on neuropathology markers in APP / PS1 Alzheimer mice. 2016:1-16. doi:10.1111/nan.12319.
- Development CD. C ELL -B ASED D RUG D EVELOPMENT , S CREENING , AND T OXICOLOGY Phase I Trial of Repeated Intrathecal Autologous Bone Marrow-Derived Mesenchymal Stromal Cells in Amyotrophic Lateral Sclerosis. 2015:590-597.
- Prabhakar S, Marwaha N, Lal V, Sharma RR, Rajan R, Khandelwal N. amyotrophic lateral sclerosis?: A pilot study Autologous bone marrow-derived stem cells in amyotrophic lateral sclerosis?: A pilot study. 2016;(September 2012). doi:10.4103/0028-3886.103185.