Stem Cells and Neurodegenerative Diseases – the Clinical Perspective
Neurodegenerative diseases like Parkinson’s disease (PD), Alzheimer’s disease (AD), Multiple Sclerosis (MS) or amyotrophic lateral sclerosis (ALS) have little to no known cure. In most cases, the therapeutic options are reduced to treating symptoms.
Stem cell therapy has an entirely different approach as it has the potential to target the underlying mechanisms of neurodegeneration. Neurodegenerative disorders are coupled with autoimmune defects of some sort, usually causing accumulations of (sub)-cellular level waste materials. These disrupt the natural intra and/or extra cell regeneration cycles in turn. Stem cells can intervene at all levels of this process.
Many different stem cell sources and approaches have been employed clinically. The prevailing source has been autologous mesenchymal stem cells (MSCs). Bone marrow and fatty tissue convince as sources for MSCs due to their availability, robustness and especially because they were found to be safe in terms of induction of neoplasms.
There is extensive research about MSCs as a therapeutic tool against neurodegenerative diseases such as Alzheimer’s disease (AD), Parkinson’s disease (PD), multiple sclerosis (MS), amyotrophic lateral sclerosis (ALS) and Huntington’s disease (HD). Two major aspect in context of their neurodegenerative abilities are of interest: their ability to transdifferentiate into neural cells under specific conditions and their neuroprotective and immunomodulatory properties. The latter is the so called paracrine effect resulting from the intrinsic environmentally responsive nature of MSCs.
Stem Cells and Neurodegenerative Diseases - the Biological Perspective
Paracrine effects have shown to be the dominant factor in neuroprotective and neuroregenerative abilities of stem cells. The effects are mediated mainly by (neuro-) trophic factors in form of cytokines, chemokines and enzymes especially in shape of extracellular vesicles like microvesicles and exosomes. This is known as the stem cell secretome (SCS).
Therefore, brain repair may be achieved (hypothetically) using the biologics secreted by mesenchymal stem cells (MSCs) alone without injecting any stem cells. This insight has dominated biological research of stem cell therapies. Whilst clinical studies for neurodegenerative diseases using stem cells still mainly imply simply injecting stem cells, the sole injecting has only little meaning in fundamental and pharmacological stem cell research anymore.
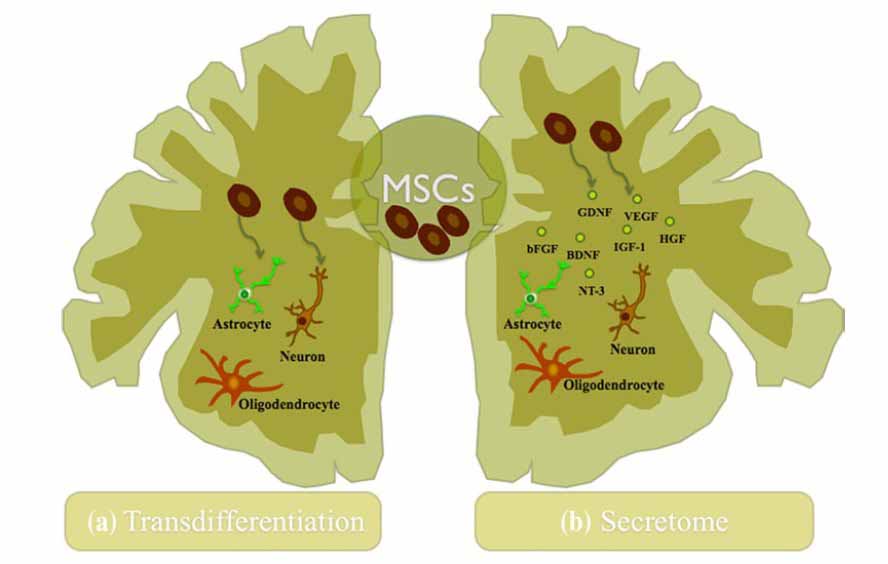
Figure 1: Mechanisms of action of MSCs in the CNS. (a) The trans-differentiation capacity of MSCs into neuronal and glial lineages both in vitro and in vivo was described as the probable explanation for their beneficial outcome after transplantation into the CNS, although this concept remains still unclear. (b) Nowadays, the trophic action of MSCs has been increasingly accepted as a new concept of regeneration of the CNS. These cells secretion of growth and neurotrophic factors has been described as an assistant in the nervous tissue regeneration through the activation/modulation of endogenous processes like the promotion of neurogenesis, angiogenesis, and immunomodulation. In this way, they contribute to the neuroprotection and regeneration of the CNS [8].
The Secretome of Stem Cells and its Effect against Neurodegeneration: Extracellular Vesicles and Soluble Factors Initiating Healing and Immune Modulation
As shown in this introduction, the stem cell secretome (SCS) of mesenchymal stem cells consists of hundreds of immune, inflammatory and paracrine factors. For several reasons, the secretome and especially the paracrine extracellular vesicles could be (part of) the solution for many neurodegenerative diseases:
- It is the essence of what stem cell therapies from the past were able to achieve in terms of positive clinical outcomes
- Now for the first time the dose can be regulated and optimized to yield a lasting response
- Since the extraction methods can be designed with high efficacy, larger quantities can be produced
- These extraction methods allow good repeatability without new required fat / bone marrow tissue
- Latest research shows, that the neurogenic niche appears to be a key research target. MSC secretome has been shown to regulate the neurogenic niche towards promoting neurogenesis
- It shows that natural factors that were involved in the formation and development of the brain and motor functions in embryonic state are not unlikely to be contained in the inter-cell communication cytokines and therefore answer to auto-immune and/or neurodegenerative diseases
- The stem cells can be designed and changed by altering either genes or simply the conditions of stress during extraction process
- Extracellular vesicles can easily cross the blood brain barrier (BBB), regardless of injection site (intranasal, intrathekal or intravenous injections)
- Stem cells` factors appear to have positive impact only: anti-inflammation, neurotrophic, neuroprotective, immune modulatory effects etc. (excepted might be Alzheimer`s disease where exosomes can both clear and favor amyloid beta plaques)
- Accumulating studies demonstrated that exosomes initiated and regulated neuroinflammation, modified neurogenic niches and neurogenesis
- None of the risks involved with injecting (cultivated) MSCs
- Allogenic use and even off the shelf use thinkable (long term, currently only under point of care, see “our code” below)
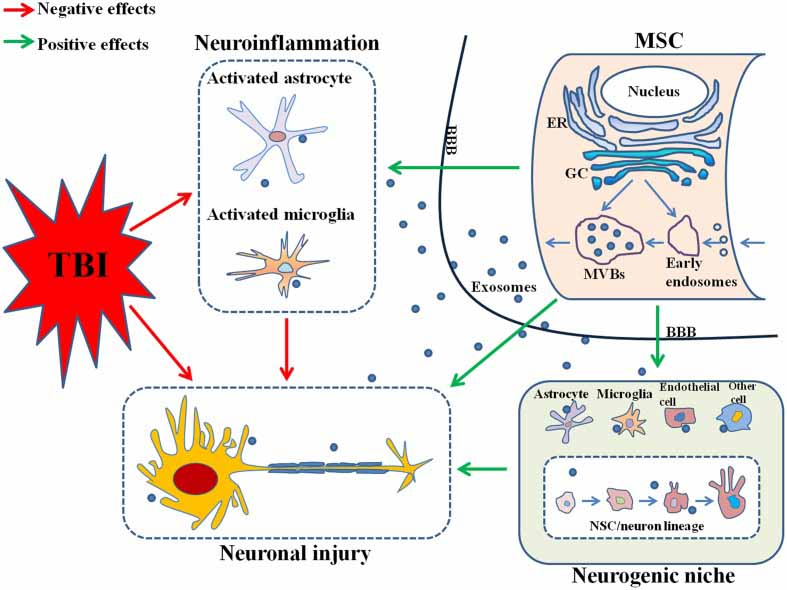
Figure 2: MSC, mesenchymal stem cell; TBI, traumatic brain injury; ER, endoplasmic reticulum; GC, Golgi complex; MVBs, multivesicular bodies; BBB, brain-blood barrier; NSC, neural stem cell [4].
References and Literature - Stem Cell-based Therapies and Neurodegenerative Diseases
- Tanna, Tanmay, and Vatsal Sachan. "Mesenchymal stem cells: potential in treatment of neurodegenerative diseases." Current stem cell research & therapy 9.6 (2014): 513-521.
- Li, Matthew D., Harold Atkins, and Tania Bubela. "The global landscape of stem cell clinical trials." Regenerative medicine 9.1 (2014): 27-39.
- Drago, Denise, et al. "The stem cell secretome and its role in brain repair." Biochimie 95.12 (2013): 2271-2285.
- Silva, Andreia M., et al. "Extracellular vesicles: immunomodulatory messengers in the context of tissue repair/regeneration." European Journal of Pharmaceutical Sciences 98 (2017): 86-95.]
- Yang, Yongxiang, et al. "MSCs-Derived Exosomes and Neuroinflammation, Neurogenesis and Therapy of Traumatic Brain Injury." Frontiers in Cellular Neuroscience 11 (2017).
- Salgado, Antonio J., et al. "Mesenchymal stem cells secretome as a modulator of the neurogenic niche: basic insights and therapeutic opportunities." Frontiers in cellular neuroscience 9 (2015).
- Kim, Hyun Ok, Seong-Mi Choi, and Han-Soo Kim. "Mesenchymal stem cell-derived secretome and microvesicles as a cell-free therapeutics for neurodegenerative disorders." Tissue Engineering and Regenerative Medicine 10.3 (2013): 93-101.
- Teixeira, Fábio G., et al. "Mesenchymal stem cells secretome: a new paradigm for central nervous system regeneration?." Cellular and Molecular Life Sciences 70.20 (2013): 3871-3882.
References and Literature - Stem Cell Secretome
- Konala, Vijay Bhaskar Reddy, et al. "The current landscape of the mesenchymal stromal cell secretome: a new paradigm for cell-free regeneration." Cytotherapy 18.1 (2016): 13-24.
- Lopez-Verrilli, M. A., et al. "Mesenchymal stem cell-derived exosomes from different sources selectively promote neuritic outgrowth." Neuroscience 320 (2016): 129-139.
- Kim, Hyun Ok, Seong-Mi Choi, and Han-Soo Kim. "Mesenchymal stem cell-derived secretome and microvesicles as a cell-free therapeutics for neurodegenerative disorders." Tissue Engineering and Regenerative Medicine 10.3 (2013): 93-101.
- Rani, Sweta, et al. "Mesenchymal stem cell-derived extracellular vesicles: toward cell-free therapeutic applications." Molecular Therapy 23.5 (2015): 812-823.
- Zhang, Xiaoyan, et al. "Mesenchymal Stem Cell-Derived Extracellular Vesicles: Roles in Tumor Growth, Progression, and Drug Resistance." Stem Cells International 2017 (2017).
- omzikova, Marina O., and Albert A. Rizvanov. "Current Trends in Regenerative Medicine: From Cell to Cell-Free Therapy." BioNanoScience (2016): 1-6.
- Zhang, Bin, et al. "Focus on extracellular vesicles: Therapeutic potential of stem cell-derived extracellular Vesicles." International journal of molecular sciences 17.2 (2016): 174.
- Katsuda T. et al. (2013). Human adipose tissue-derived mesenchymal stem cells secrete functional neprilysin-bound exosomes. Scientific reports, 3, 1197.
- Pusic A. D. et al. (2014). IFNγ-stimulated dendritic cell exosomes as a potential therapeutic for remyelination. Journal of neuroimmunology, 266(1), 12-23.
- Maumus M, Jorgensen C, Noël D (2013) Mesenchymal stem cells in regenerative medicine applied to rheumatic diseases: Role of secretome and exosomes. Biochimie 95:2229–2234. doi: 10.1016/j.biochi.2013.04.017
- Drago D, Cossetti C, Iraci N, et al (2013) Biochimie The stem cell secretome and its role in brain repair. Biochimie 95:2271–2285. doi: 10.1016/j.biochi.2013.06.020
- Sevivas N, Teixeira FG, Portugal R, et al (2016) Mesenchymal Stem Cell Secretome: A Potential Tool for the Prevention of Muscle Degenerative Changes Associated With Chronic Rotator Cuff Tears. Am J Sports Med. doi: 10.1177/0363546516657827
- Hs K (2016) Mesenchymal Stem Cells vs . Mesenchymal Stem Cell Secretome for Rheumatoid Arthritis Treatment. 1:1–2.
- Maumus M, Jorgensen C, Noël D (2013) Mesenchymal stem cells in regenerative medicine applied to rheumatic diseases: Role of secretome and exosomes. Biochimie 95:2229–2234. doi: 10.1016/j.biochi.2013.04.017
- Kapur SK, Katz AJ (2013) Biochimie Review of the adipose derived stem cell secretome. Biochimie 95:2222–2228. doi: 10.1016/j.biochi.2013.06.001
- Ranganath SH, Levy O, Inamdar MS, Karp JM (2012) Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell 10:244–258. doi: 10.1016/j.stem.2012.02.005
- Tran C, Damaser MS (2015) Stem cells as drug delivery methods: Application of stem cell secretome for regeneration. Adv Drug Deliv Rev 82:1–11. doi: 10.1016/j.addr.2014.10.007
- Zimmerlin L, Park TS, Zambidis ET, et al (2013) Mesenchymal stem cell secretome and regenerative therapy after cancer. Biochimie 95:2235–2245. doi: 10.1016/j.biochi.2013.05.010
- Calamia V, Lourido L, Fernandez-Puente P, et al (2012) Secretome analysis of chondroitin sulfate-treated chondrocytes reveals its anti-angiogenic, anti-inflammatory and anti-catabolic properties. Arthritis Res Ther 14:R202. doi: 10.1186/ar4040
- Ranganath SH, Levy O, Inamdar MS, Karp JM (2012) Review Harnessing the Mesenchymal Stem Cell Secretome for the Treatment of Cardiovascular Disease. Stem Cell 10:244–258. doi: 10.1016/j.stem.2012.02.005
- Teixeira FG, Carvalho MM, Sousa N, Salgado AJ (2013) Mesenchymal stem cells secretome: A new paradigm for central nervous system regeneration? Cell Mol Life Sci 70:3871–3882. doi: 10.1007/s00018-013-1290-8
- Kapur SK, Katz AJ (2013) Review of the adipose derived stem cell secretome. Biochimie 95:2222–2228. doi: 10.1016/j.biochi.2013.06.001
- Chang C-P, Chio C-C, Cheong C-U, et al (2013) Hypoxic preconditioning enhances the therapeutic potential of the secretome from cultured human mesenchymal stem cells in experimental traumatic brain injury. Clin Sci (Lond) 124:165–76. doi: 10.1042/CS20120226
- Salgado AJ, Sousa JC, Costa BM, et al (2015) Mesenchymal stem cells secretome as a modulator of the neurogenic niche: basic insights and therapeutic opportunities. Front Cell Neurosci 9:1–18. doi: 10.3389/fncel.2015.00249
- Ahmed NE-MB, Murakami M, Hirose Y, Nakashima M (2016) Therapeutic Potential of Dental Pulp Stem Cell Secretome for Alzheimer’s Disease Treatment: An In Vitro Study. Stem Cells Int 2016:8102478. doi: 10.1155/2016/8102478
- Bhaskar V, Konala R, Mamidi MK, et al (2016) The current landscape of the mesenchymal stromal cell secretome : A new paradigm for cell-free regeneration. Cytotherapy 18:13–24. doi: 10.1016/j.jcyt.2015.10.008
- Malda J, Boere J, van de Lest C, et al (2016) Extracellular vesicles - new tool for joint repair and regeneration - IN PRESS. Nat Rev Rheumatol 12:243–249. doi: 10.1038/nrrheum.2015.170
- Lener T, Gimona M, Aigner L, et al (2015) Applying extracellular vesicles based therapeutics in clinical trials Á an ISEV position paper. 1:1–31.
- Dostert G, Mesure B, Menu P, Velot É (2017) How Do Mesenchymal Stem Cells Influence or Are Influenced by Microenvironment through Extracellular Vesicles Communication ? 5:1–7. doi: 10.3389/fcell.2017.00006
- Joshi P, Benussi L, Furlan R, et al (2015) Extracellular vesicles in Alzheimer’s disease: Friends or foes? focus on Aβ-vesicle interaction. Int. J. Mol. Sci. 16:4800–4813.
- Gao T, Guo W, Chen M, et al (2016) Extracellular Vesicles and Autophagy in Osteoarthritis.
- Katsuda T, Ochiya T (2015) Molecular signatures of mesenchymal stem cell-derived extracellular vesicle-mediated tissue repair. Stem Cell Res Ther 6:212. doi: 10.1186/s13287-015-0214-y
- Lener T, Gioma M, Aigner L, et al (2015) Applying extracellular vesicles based therapeutics in clinical trials - an ISEV position paper. J Extracell Vesicles 4:1–31. doi: 10.3402/jev.v4.30087
- Xu Y, Guo S, Wei C, et al (2016) The Comparison of Adipose Stem Cell and Placental Stem Cell in Secretion Characteristics and in Facial Antiaging.
- Buul GM Van, Villafuertes E, Bos PK, et al (2012) Mesenchymal stem cells secrete factors that inhibit in fl ammatory processes in short-term osteoarthritic synovium and cartilage explant culture. Osteoarthr Cartil 20:1186–1196. doi: 10.1016/j.joca.2012.06.003
- Baglio SR, Pegtel DM, Baldini N (2012) Mesenchymal stem cell secreted vesicles provide novel opportunities in ( stem ) cell-free therapy. 3:1–11. doi: 10.3389/fphys.2012.00359
- Anderson JD, Pham MT, Contreras Z, et al (2016) Mesenchymal stem cell-based therapy for ischemic stroke. Chinese Neurosurg J 2:36. doi: 10.1186/s41016-016-0053-4
- Biology C, Cell R, Eye N, Institutes N (2017) Bone Marrow-Derived Mesenchymal Stem Cells-Derived Exosomes Promote Survival of Retinal Ganglion Cells Through miRNA-Dependent Mechanisms. 1273–1285.